Questions

Choosing the right buffers and solvents is critical in LC-MS because the entire mobile phase directly enters the mass spectrometer. Compatibility with the ionization source (like ESI or APCI) is essential for high-quality data.
1. Why Volatile Buffers are Essential
Non-volatile salts, such as phosphate buffers, are unsuitable for LC-MS. As the mobile phase evaporates, these salts leave a residue that can:
Contaminate and clog the ion source.
Cause signal suppression.
Increase background noise.
Necessitate frequent and costly maintenance.
Volatile buffers are the solution. They provide stable pH control while being easily removed in the gas phase.
Benefits of Volatile Buffers:
High Sensitivity: They evaporate cleanly, allowing analytes to ionize efficiently without interference.
Low Background: Complete evaporation minimizes background ions, leading to a cleaner spectrum and better signal-to-noise.
System Stability: They prevent the residue buildup that plagues systems using non-volatile salts.
Common Volatile Buffers:
Ammonium Acetate (pH range ~4-6)
Ammonium Formate (pH range ~3-6)
Formic Acid / Acetic Acid (for acidic conditions)
Ammonium Hydroxide (for basic conditions)
2. Why Non-Polar Solvents Should Be Avoided
The choice of organic modifier is equally important. Non-polar solvents (like hexane or toluene) are not recommended for typical reversed-phase LC-MS for several reasons:
Poor Miscibility: Most LC-MS mobile phases are aqueous. Non-polar solvents are immiscible with water, which can cause phase separation.
Low Volatility & Unstable Spray: They do not evaporate efficiently in the MS source, leading to an unstable spray, a noisy baseline, and system contamination.
Ion Suppression: Their low dielectric constant is not conducive to good ion formation in ESI, resulting in poor signal intensity.
Safety Concerns: They often have higher toxicity and flammability.
3. Preferred Solvents for LC-MS
The most commonly used solvents are polar and volatile, such as Methanol and Acetonitrile.
These are ideal because they are:
Fully miscible with water.
Highly volatile, ensuring easy removal in the ion source.
Able to produce stable electrospray droplets, which promotes efficient ionization.
#PharmaceuticalAnalysis #MethodDevelopment #Principle #LCMS #UHPLC #HPLC #GC #GCMS #Volatile_Buffers #Non_polar_solvents #USP #IP #WHO #CDCSO #ICH #MHRA #AnalyticalChemistry #Solactivity #DrugDevelopment #Chemist #validation #science #pharma #GLP #SCIENCE #analytical #scientist #knowledge #sharing
No answers yet.

When developing an HPLC method, we often focus on column chemistry, mobile phase composition, and detection. But what happens when the compound itself is chiral?
Enantiomers — mirror-image molecules — behave identically in most achiral environments, which means they often co-elute as a single peak on standard C18 or C8 columns. In biological systems, however, their behavior can be dramatically different:
One enantiomer may provide the therapeutic effect.
The other may be inactive, or even harmful.
This is why regulatory agencies (ICH, FDA, EMA) require chiral separation and quantification during method development and validation. Enantiomeric purity is not only a regulatory requirement but also essential for patient safety and drug efficacy.
Strategies for Chiral Separation in HPLC:
Chiral Stationary Phases (CSPs) – polysaccharide, cyclodextrin, protein, or Pirkle-type columns that enable selective interactions.
Chiral Mobile Phase Additives (CMPAs) – such as cyclodextrins forming transient diastereomeric complexes.
Indirect Approach (Derivatization) – converting enantiomers into diastereomers with chiral reagents (e.g., Marfey’s, Mosher’s) for separation on achiral columns.
Key Considerations in Method Development:
Screening multiple CSPs to identify the best selectivity.
Selecting the appropriate elution mode (normal-phase, reversed-phase, polar organic).
Optimizing pH and temperature to improve resolution.
Ensuring scalability for both analytical and preparative applications.
In modern pharmaceutical analysis, chirality is more than just a separation challenge — it is a critical quality attribute that guarantees both drug safety and efficacy.
For professionals in analytical R&D and QC, developing strong expertise in chiral HPLC method development is essential. Chirality is not just chemistry; it is directly linked to patient safety.
#HPLC #Chirality #MethodDevelopment #AnalyticalChemistry #Pharma #DrugSafety #QualityControl
No answers yet.

One of the most important choices in chromatography method development is deciding between Normal-Phase (NP) and Reversed-Phase (RP) HPLC. The following comparison outlines the main differences:
Normal-Phase HPLC
The stationary phase is more polar than the mobile phase.
Common column types include Silica, Amino (NH2), Diol, and Cyano (CN).
The mobile phase usually consists of non-polar solvents such as hexane or ethyl acetate.
Polar compounds are retained for a longer time.
Reversed-Phase HPLC
The stationary phase is less polar than the mobile phase.
Typical columns include ODS (C18), C8, C4, Phenyl, and Cyano (CN).
The mobile phase often contains polar solvents such as water or acetonitrile.
Non-polar compounds are retained for a longer time.
The retention order of analytes is essentially reversed when switching between NP and RP systems—this principle lies at the core of chromatographic separation.
Choosing the appropriate phase depends on factors such as analyte polarity, sample solubility, and the desired separation outcome.
#HPLC #Chromatography #AnalyticalChemistry #Pharmaceuticals #PharmaIndustry #DrugDevelopment #SeparationScience #ResearchAndDevelopment #LabWork #QualityControl #QC #MethodDevelopment #AnalyticalMethods #Biopharma #ChemicalAnalysis #PharmaResearch #ChromatographicSeparation
No answers yet.
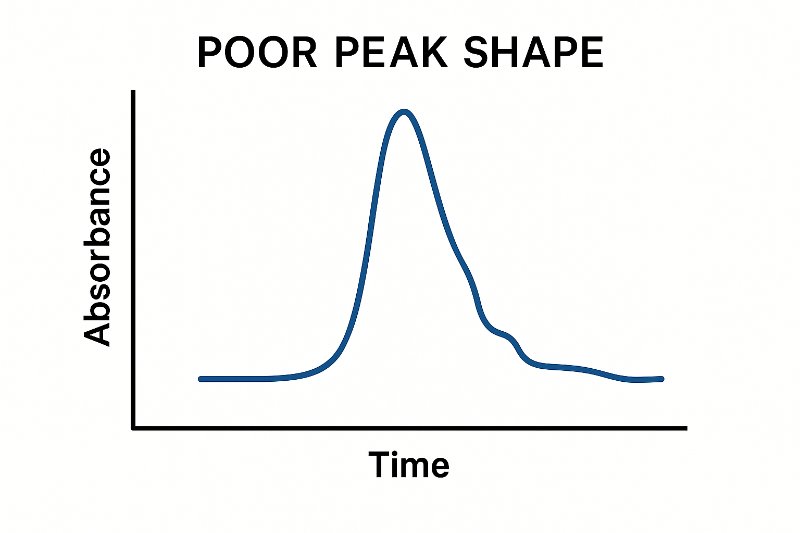
Think about the pKa of basic drugs – how are they charged at low pH?
Consider the stationary phase – what happens when silanol groups are not masked?
Peak fronting/tailing + retention issues = possible secondary interactions or ionization mismatch.
Which condition increases interaction with residual silanols?

“Column selection isn’t just one step in method development—it’s the cornerstone of achieving accurate, reliable separations.”
Here are essential tips to guide your column selection process:
1. Understand Your Analyte
Begin with the basics: polarity, pKa, molecular weight, solubility, and chemical stability. For polar compounds, HILIC is ideal. For non-polar compounds, C18 or phenyl columns are typically preferred.
2. Choose the Right Stationary Phase
Start with C18 (ODS), the go-to choice for general applications. If needed, explore C8, phenyl-hexyl, cyano, or polar-embedded phases to fine-tune selectivity.
3. Mind Column Dimensions
Length: Longer columns improve resolution but increase analysis time.
Diameter: Narrow-bore columns (e.g., 2.1 mm) reduce solvent consumption.
Particle Size: Smaller particles (1.7–3 µm) enhance efficiency, but increase system back pressure.
4. Assess pH Stability
When working in acidic or basic conditions, opt for columns with wide pH stability ranges such as hybrid-silica or polymer-based columns.
5. Understand Endcapping
Endcapped columns minimize silanol interactions, which is especially useful for basic analytes. For stronger polar interactions, non-endcapped columns can be advantageous.
6. Ensure System Compatibility
Make sure the selected column is compatible with your detector, mobile phase, and system pressure—particularly when using UHPLC.
7. Use Orthogonal Screening
Test multiple columns with distinct selectivities. This parallel approach speeds up development and improves robustness.
8. Leverage Existing Knowledge
Explore pharmacopeial methods, published research, and manufacturer application notes—many solutions already exist.
Pro Tip: Keep a detailed column log and performance database. It’s a game-changer for future troubleshooting and method reuse.
Thoughtful column selection accelerates development, reduces trial-and-error, and builds regulatory confidence.
Post Credit: Dr. Nilanjana Rana
#HPLC #AnalyticalChemistry #Pharma #MethodDevelopment #QualityControl #Chromatography #UHPLC #ColumnSelection #RnD #HPLCTips
No answers yet.

